Vernal’s World Vaccine Congress Poster
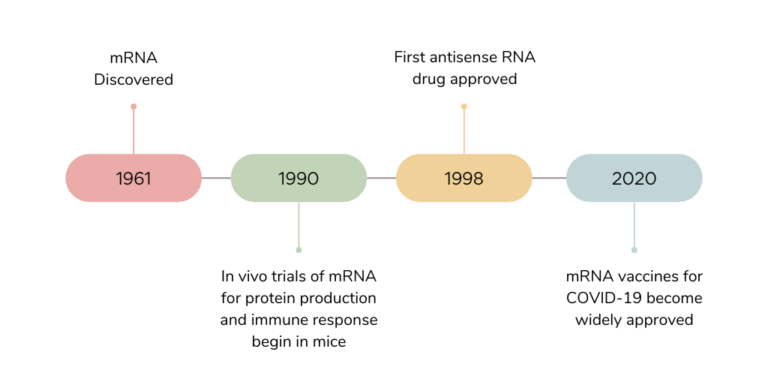
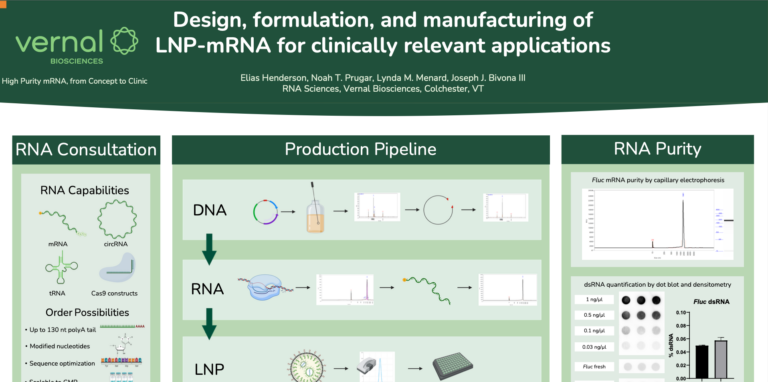
Sorry we missed you at the World Vaccine Congress in DC this April (2023). As previously mentioned, we showcased our poster providing information on the efficacy of LNP-mRNA using Sars-Cov-2 spike protein to demonstrate. We encourage you to download our…