By Julia Sakamoto, Marketing Specialist
The development of mRNA vaccines has been ongoing since the late 80s, but it wasn’t until the COVID-19 pandemic that this technology became widely recognized. Instead of using an antigen to trigger the body’s immune system to create a new antibody, mRNA can give direct instructions on how to fight a certain virus or disease. Advancing this technology has taken time as researchers initially worked with smaller data sets and minimal funding. Another limiting factor was that earlier generations of the mRNA vaccines were unstable and degraded too quickly in vivo. While capping has allowed stability of synthetic mRNA and enabled mRNA-based therapeutic applications, the use of lipid nanoparticles, or (LNP)-formulated mRNA, has been rapidly developed and deployed in response to the COVID-19 pandemic, and now to treat various diseases.
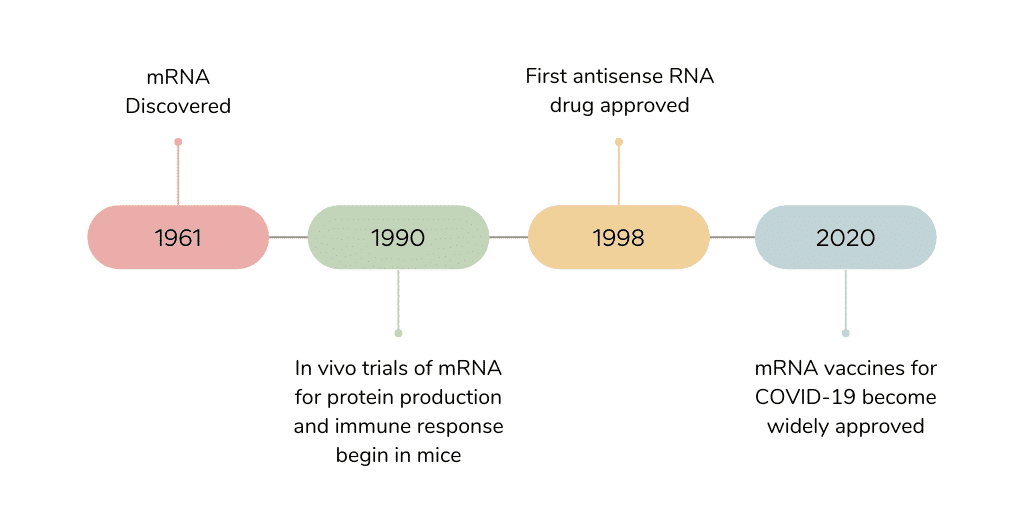
Figure 1. Brief timeline of mRNA vaccines
LNP consists of a fatty layer encasing the mRNA and protecting it from immunogenicity. To break it down further, there is a PEGylated lipid, ionizable lipid, helper lipid, and cholesterol (see Figure 2). The PEGylated lipid enhances LNP stability while providing a neutral surface charge. Meanwhile, the positively charged ionizable lipids help with mRNA release through LNP destabilization after entering the cell. Helper lipids provide further delivery stability while cholesterol adds to the lipid shell structure and membrane fusion. Considering its effective use and potent adjuvant activity, LNP has helped with bringing mRNA medicine to the clinical stage.

Figure 2. Diagram of LNP encasing mRNA
The expansion of clinically-approved mRNA medicines have enabled researchers to move into a new wave of possibilities for full length proteins. Experimental mRNA medicines are emerging for cancer1,2, heart disease3, and autoimmune disorders4. Additionally, the ability to rapidly design and manufacture LNP-mRNA vaccines has been vital to reducing morbidity during the COVID-19 pandemic. As the use cases and clinical trials for mRNA medicines grows, so too must the number of trusted manufacturing partners to produce therapeutics from idea conception through clinical trials.
To display Vernal Biosciences’ capabilities in producing high-quality mRNA and LNP-mRNA, we first manufactured two mRNA variants of the SARS-CoV-2 spike protein. The mRNAs were formulated and packaged in-house in LNPs consisting of clinically relevant ionizable lipids and injected intramuscularly into BALB/C mice twice over 15 days in a prime-boost paradigm. The serum was collected from the immunized mice and analyzed by ELISA. We show that the mice elicited a potent immune response in the vaccinated mice: the antibody titers were equivalent to those of peer-reviewed, preclinical studies of FDA approved COVID-19 vaccines5,6,7.
Stay tuned as we will be providing more details in our newsletter as we get ready for the World Vaccine Congress in Washington DC. Please come visit our booth (#905) in April to find out more about how we can help with your mRNA-LNP formulations.
Contact us HERE.
References
1. Di Trani, C. A. et al. Advances in mRNA-based drug discovery in cancer immunotherapy. Expert Opin. Drug Discov. 17, 41–53 (2022).
2. Emiliano Ferdows, B. et al. RNA cancer nanomedicine: nanotechnology-mediated RNA therapy. Nanoscale 14, 4448–4455 (2022).
3. Collén, A. et al. VEGFA mRNA for regenerative treatment of heart failure. Nat. Rev. Drug Discov. 21, 79–80 (2022).
4. Flemming, A. mRNA vaccine shows promise in autoimmunity. Nat. Rev. Immunol. 21, 72–72 (2021).
5. Corbett, K. S. et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 586, 567–571 (2020).
6. Vogel, A. B. et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature 592, 283–289 (2021).
7. Ying, B. et al. Boosting with variant-matched or historical mRNA vaccines protects against Omicron infection in mice. Cell 185, 1572-1587.e11 (2022).
