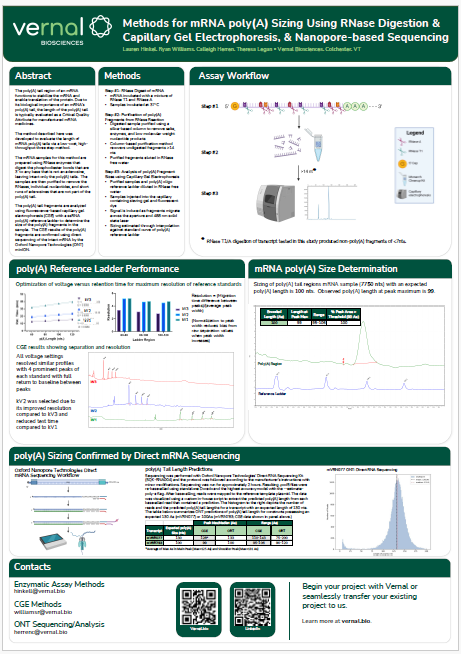
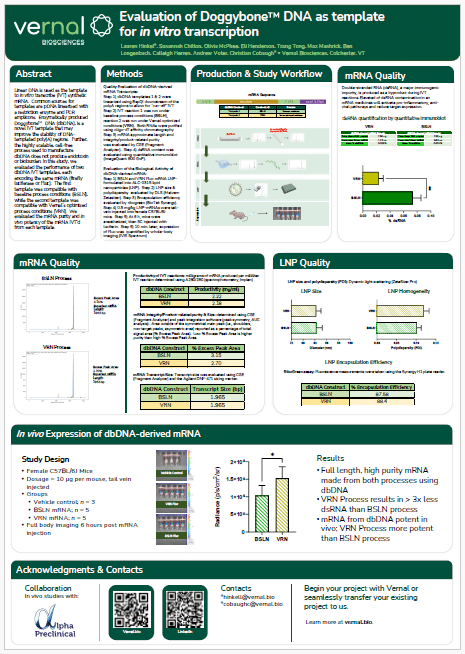
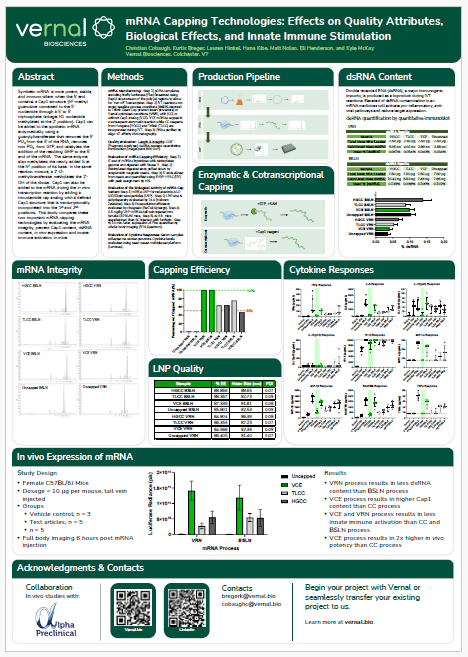
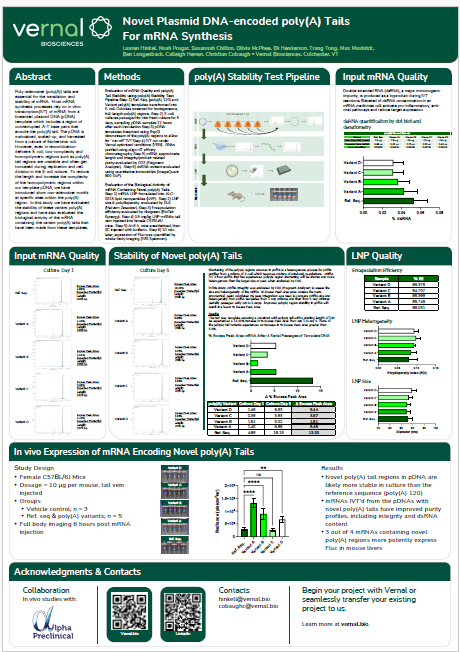
The mRNA Monthly—August 2024 Newsletter
Rapid Single Epitope mRNA Production This article presents a synthetic DNA template (SDT) process for manufacturing single epitope mRNA, offering a flexible, cost-efficient, and rapid alternative to traditional plasmid DNA templates (PDT). Read the article to explore what can be…