Scalable Next-Generation LNP
Flexible Lipid Nanoparticle Formulations of mRNA
Vernal Biosciences offers scalable lipid nanoparticle (LNP) formulation services using a growing list of lipid chemistries to keep your mRNA program advancing through cell and animal studies. Our formulation mixing and purification technologies scale your project from small to large animal studies, and ultimately to clinical trials. Our GMP operations have all the technology you need for clinical manufacturing and sterile fill-finish of LNP-mRNA.
Preclinical LNP Manufacturing and Formulation Services
Combined with our high purity mRNA, Vernal’s LNP Manufacturing and Formulation Service will help accelerate your programs into preclinical success. Vernal’s LNP Formulation scientists will help you choose research-ready lipid chemistries that will keep your LNP-mRNA formulation stable during storage and protect it from degradation after it is injected, as well as reach your organs and cells of interest. We will work with you to determine the dosing, including dose level and interval, volume of injection, and route of administration (ROA) for your LNP-mRNA formulation. The LNP formulations that Vernal manufactures can be injected through intravenous, subcutaneous, intramuscular, intraventricular, and intratumoral ROAs.
Once the details of your LNP-mRNA application are finalized, our scientists will use a scale-appropriate mixing technology to manufacture your LNP-mRNA formulation to the specifications, while also adding a cryoprotectant. Before releasing and shipping the LNP-mRNA formulation, release testing of the final products will be performed to ensure that you receive high purity, potent finished material.
Our research using lipid nanoparticle formulations for liver & lungs (IV), and vaccines (IM)
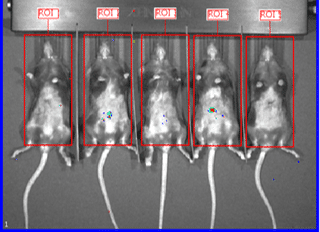
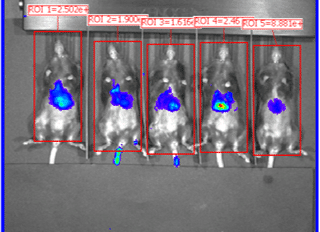
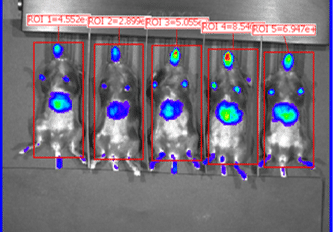
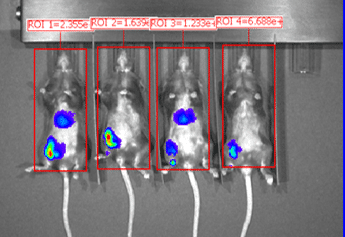

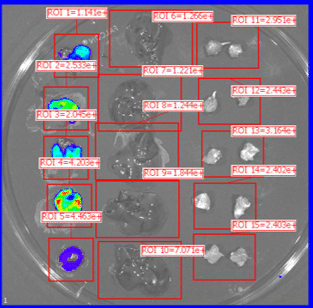
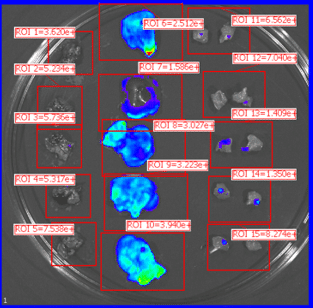
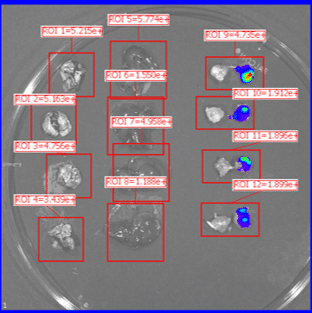
Organs harvested for LNP formulation and biodistribution study from left to right: lungs, liver, and muscle
Similar to real estate, drug delivery of mRNA medicines is all about location, location, and location. Fortunately, Vernal has worked out LNP compositions and clinically-relevant ROAs that can deliver your mRNA payloads to organs with high value therapeutic targets. Our biodistribution studies have proven that our formulation technologies offer effective LNP delivery to the location of interest and are highly bioactive once delivered. In our first mouse biodistribution study, we targeted the lungs by injecting LNP-mRNA Formulation #1 intravenously. We also targeted liver and muscle using LNP-mRNA Formulation #2, via intravenous and intramuscular ROAs. The LNP payload consisted of an mRNA encoding the firefly luciferase (FLuc) protein, a reporter gene that lights up its substrate, luciferin (injected separately), to show where the LNPs have been delivered and where the FLuc has been expressed from the mRNA. In vivo imaging shows that the LNP-mRNA formulations are distributed to the targeted organs and are active at those locations.
In addition to producing highly potent LNP formulations, Vernal offers quality controlled mRNA through platform processes that enable us to maintain purity. We are committed to offering extensive support for your project throughout its entire journey, from the initial discovery phase using our research-use manufacturing to the advanced clinical trials stage with our GMP manufacturing. It is never too early to collect and contextualize process data, which helps create pathways directly from research-use manufacturing to GMP manufacturing. All of Vernal’s process technologies and key projects leverage a Quality by Design software platform that enables a deep understanding of the relationships between the critical process parameters and quality attributes, allowing seamless, rapid technical transfers between the research-use manufacturing operations and GMP manufacturing operations.
For LNP manufacturing, Vernal has scaled the purity of our LNP formulations through a variety of pumping and mixing technologies including microfluidics, T-mixing, syringe pumps, and large-scale, constant flow HPLC pumps. All of our mixing kits are single-use to avoid cross-contamination, and we are able to fabricate custom mixing geometries. The controllers for mixing allow us exquisite control over volumes and flow rates to ensure proper mixing, encapsulation efficiency, particle size, and uniformity. Post-mixing quenching and dilution occurs either in-line or almost immediately after mixing, followed by a final buffer exchange and concentration step. Our QC lab keeps close tabs on all operations, not only verifying the raw materials, but also underwriting the quality of intermediates and finished products using customized methods.
The commercial success of mRNA-based vaccines has led to growing interest in creating therapies and vaccines that use lipid nanoparticles for mRNA delivery to the cell to produce proteins. Another advanced technology uses lipid nanoparticles for gene delivery. Gene editing technology has the potential to modify gene expression for treatment of disease, such as inherited disorders and cancers.
To avoid constraints around payload size and immunogenicity, increasingly, the precision nuclease (e.g., a Cas protein) is delivered non-virally (i.e., LNP) with an mRNA payload that encodes the nuclease. Once the target cell gets transfected by the LNP-mRNA, the mRNA is almost immediately translated into protein, whereupon it can grab onto the co-transfected sgRNA (in the case of CRISPR-Cas), translocate to the nucleus, and edit the genome. Unlike more persistent sources of the nuclease (e.g. pDNA or AAV), the transient expression of the nuclease from mRNA reduces off-target editing. Since the LNP is not immunogenic, unlike the case with AAV-delivered gene editing, LNP delivery can be used to dose-to-effect, treating the animal or patient repeatedly until therapeutic gene editing has been reached. For RNA-guided nucleases, Vernal has worked out how to co-formulate sgRNAs and mRNAs into all of the lipid chemistries that we offer.
As preclinical research and clinical trials using LNP-mRNA treatments advance, there is a growing need for access to high-quality mRNA and lipid nanoparticle mRNA formulations. Vernal Biosciences provides on-demand services to design, manufacture, and formulate LNP-mRNA for all stages of research and development.
To validate our LNP-mRNA formulation and manufacturing platform technologies further, we manufactured two mRNA COVID-19 vaccine mimics (VRN-S-1 and VRN-S-2). One mimics Moderna’s COVID-19 vaccine, including the same mRNA sequence, uridine substitutions, and lipid chemistry used by Moderna (SM-102). The other replicates Pfizer-BioNTech’s COVID-19 vaccine, including the same mRNA sequence, uridine substitutions, and lipid chemistry as Pfizer (ALC-0315). The particles from both LNP-mRNA formulations were sized < 100 nm, with polydispersity indices both below 0.1 and encapsulation efficiency ranging from 94% to 96%.
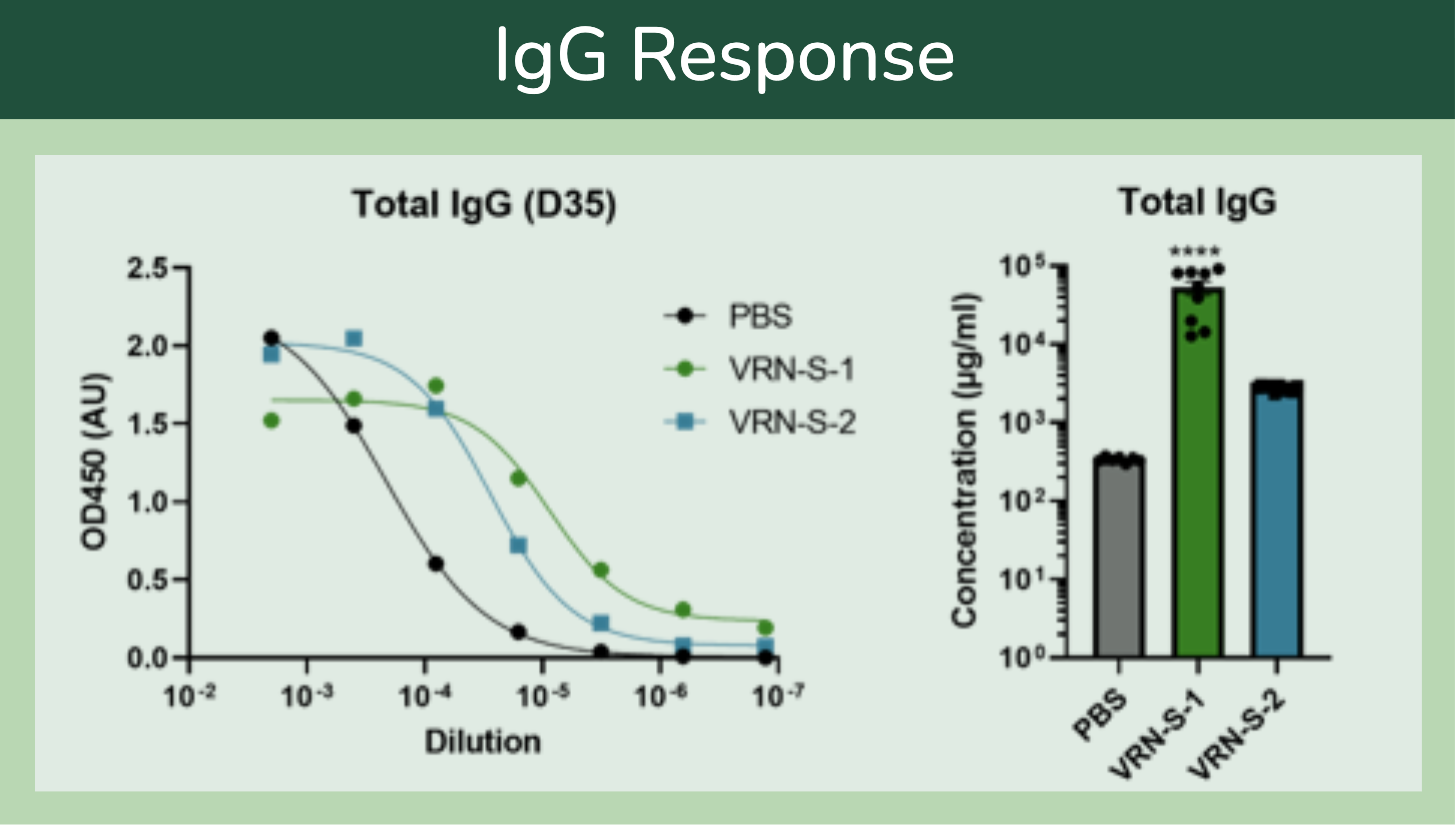
After injection into mice using a typical prime-boost intramuscular injection regimen using, immune responses were evaluated by the sera for total IgG antibody concentration as well SARS-CoV-2 S protein-specific IgG. Both VRN-S-1 and VRN-S-2 induced high levels of total IgG and S protein-specific IgG, with results comparable to the previously reported sera titer results for commercially available COVID-19 LNP-mRNA vaccines. These results demonstrate that Vernal Biosciences manufactures high-purity, high potency LNP-mRNA that results in the target biological activity. We have proactively derisked these complex manufacturing workflows so that you do not have to. Instead, you can jumpstart your preclinical, and eventually clinical programs, using our know-how.
Want to learn more?
Custom LNP development and formulation fit for your purpose

One-stop shopping: design, mRNA, LNP-mRNA

All projects at all stages incorporate our quality and regulatory experience
Flexible lipid options allow you to perform proper drug discovery on your entire drug product

Formulation services designed to scale & transition with your project all the way to clinical trials

Process development incorporates Quality by Design principle
Request a Consultation
"*" indicates required fields
Why Vernal Biosciences?
Vernal Biosciences is an mRNA CDMO democratizing the use of mRNA, LNP-mRNA, and associated services such as process development and quality control methods. Vernal’s quality-by-design driven platform technologies across the entire value chain of mRNA medicines will provide your project with a de-risked, advanced staging ground for research and clinical supply. Our on-demand, high-purity supply and services provides you with the flexibility to perform proper drug discovery and development without risking the buildout of costly teams and facilities. This transactional model and commitment to maintaining quality across all scales of mRNA and LNP-mRNA will allow your organization to focus on other high-value operations such as target biology, drug discovery, clinical development, and marketing. mRNA and LNP-mRNA from Vernal Biosciences will support all of your R&D, regardless of your mRNA use case.
