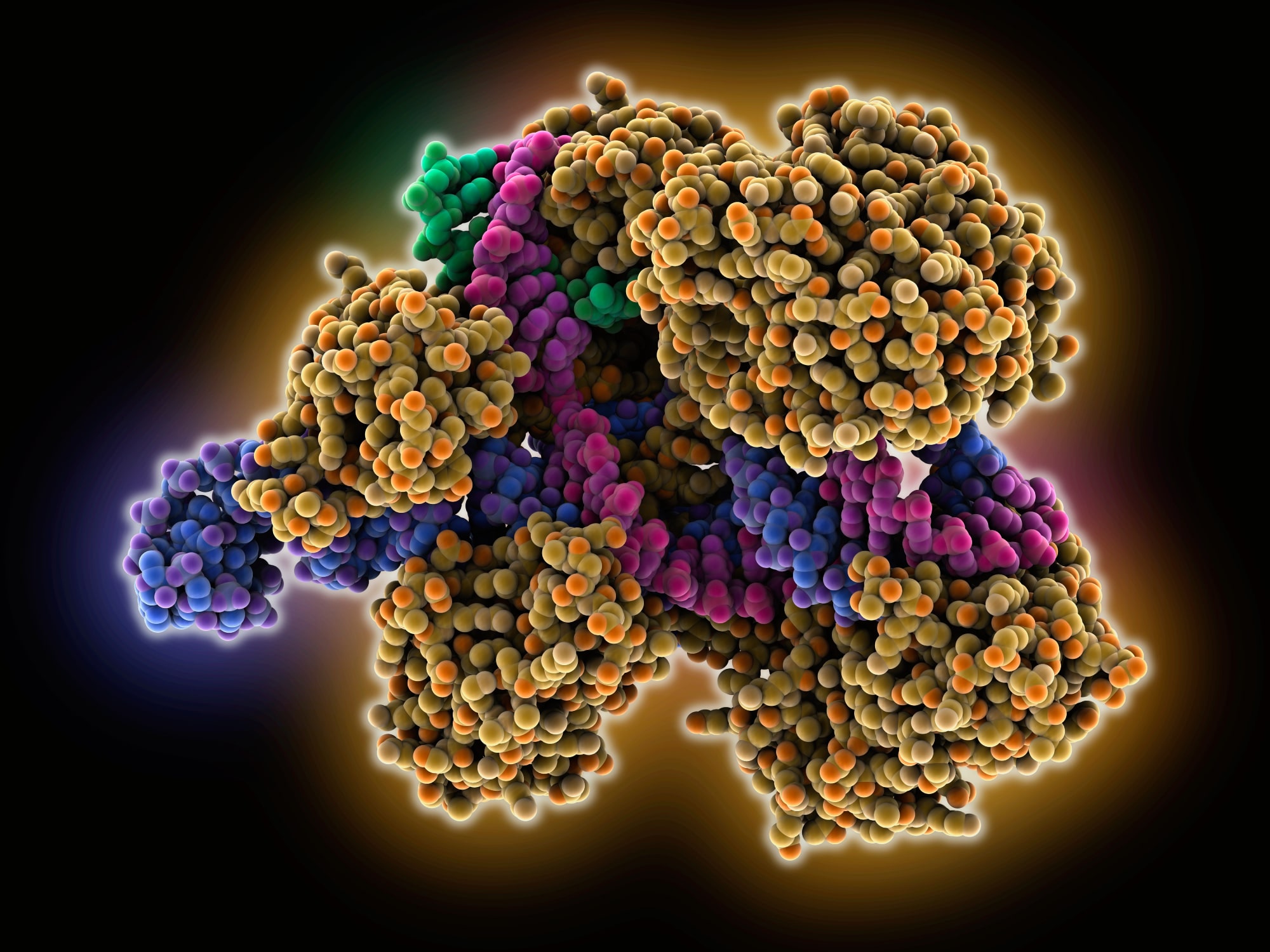
CRISPR-Cas9 RNA-DNA complex. Structure of Cas9 endonuclease (yellow-orange) complexed with a guide RNA (blue), a target DNA (pink) and a non-target DNA (green).
The revolutionary CRISPR-Cas gene editing technology has unlocked unprecedented possibilities in genome engineering, gene therapy, and cell therapy. The choice between using Cas9 mRNA or Cas9 protein becomes crucial. One has to look no further than Intellia’s remarkable safety and efficacy data from early stage clinical trials for in vivo CRISPR-Cas9 gene editing to grasp the potential of mRNA in CRISPR-Cas9 therapies. With recent improvements to mRNA manufacturing technologies, starting with sequence design, companies like Vernal Biosciences have added much needed capacity to provision all gene editing needs with high purity manufactured mRNA.
In this blog post, we will explore the key reasons why Cas9 mRNA is fast becoming the preferred approach for delivering Cas9.
Increased Potency: Cas9 mRNA ensures efficient delivery and potent, yet transient expression of Cas9 protein within target cells. The Cas9 can be introduced into cells simultaneously with sgRNA. Compared to pDNA Cas9 or viral genome-encoded Cas9, once in the cytoplasm, Cas9 mRNA is immediately translated into protein without having to be transcribed and exported from the nucleus. Once translated, the Cas9 protein will load the sgRNAs and transit the nuclear membrane through the nuclear localization signal encoded by the mRNA onto the Cas9, maximizing gene editing rates.
Flexibility in Delivery Methods: Depending on the cell type or the route of administration, Cas9 mRNA can be delivered using various methods, such as polymeric or cationic transfection reagents, lipid nanoparticles (LNP), or electroporation, facilitating cell transfection and precise targeting of cells and desired tissues or organs. This flexibility enhances Cas9 mRNA adaptability in diverse experimental and clinical scenarios. RNP, on the other hand, cannot be directly injected into lab animals or humans, and in the case of cell therapies, must be electroporated into the cells. Electroporation is not considered to be as scalable as transfection and is also more toxic to cells, reducing the overall process yields.
Temporal Control: Nature has created mRNA to provide transient levels of control of protein expression. In the case of gene editing, this is helpful, as pDNA Cas9 or viral genome-encoded Cas9 provides more persistent levels of protein expression which can raise the risk of off-target gene editing effects. On the other hand, because the mRNA has a longer half-life than RNP, it will usually result in a higher rate of gene editing. For most applications, the mRNA approach to Cas9 provides the optimal duration of expression creating the optimal balance between gene editing rates and off-target effects.
Lower Immunogenicity: Direct delivery of exogenous protein often exposes the risk of triggering immune response, leading to subsequent treatments. High-purity mRNA, recognized as a natural cellular component, reduces immune reactions, making it more suitable for repeated administration in vivo and enabling the ability to dose to the desired clinical effect. Furthermore, the use of modified mRNA has further reduced the risk of innate immune activation and an inflammatory response. The low immunogenicity of LNP-mRNA mediated CRISPR-Cas9 gene editing offers hope for durable and stable gene correction for patients.
Lower cost of Manufacturing: Manufacturing of mRNA is more cost-effective and has a shorter turnaround time than manufacturing of proteins or viral products. Manufacturers of recombinant proteins often face challenges to scale up their production capacity when scaling up from pilot to large volume bioreactors. mRNA is produced by in vitro transcription (IVT) through a highly scalable enzymatic process, allowing a smaller footprint with lower capital and labor cost.
mRNA-mediated Cas9 gene editing offers many advantages over RNP, pDNA, and viral genome-encoded Cas9 methods. These advantages are making Cas9 mRNA the preferred choice for achieving safe, precise, and controlled gene editing while avoiding immunogenicity.
Vernal is now offering SpCas9 mRNA to support your research in gene editing. We also have the GMP capacity to rapidly execute mRNA projects from research use to clinical.
