Vernal Scales Up mRNA Production Successfully
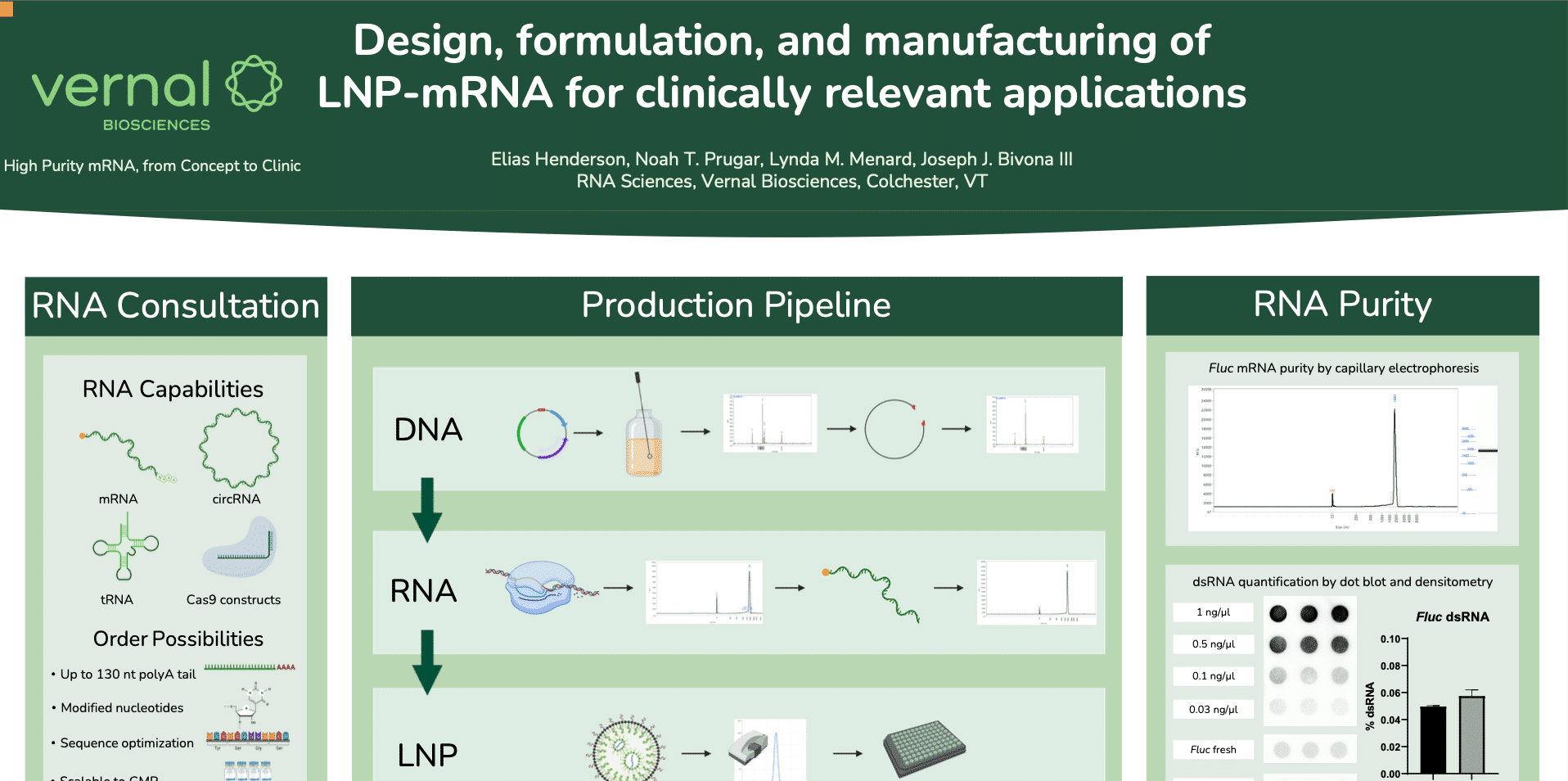
Vernal has successfully demonstrated its ability to manufacture high quality mRNA using CGMP-compliant platform. We now offer the first integrated platform for CGMP production of MCB, pDNA, mRNA, and LNP at our GMP facility. Contact us today to reserve your GMP…